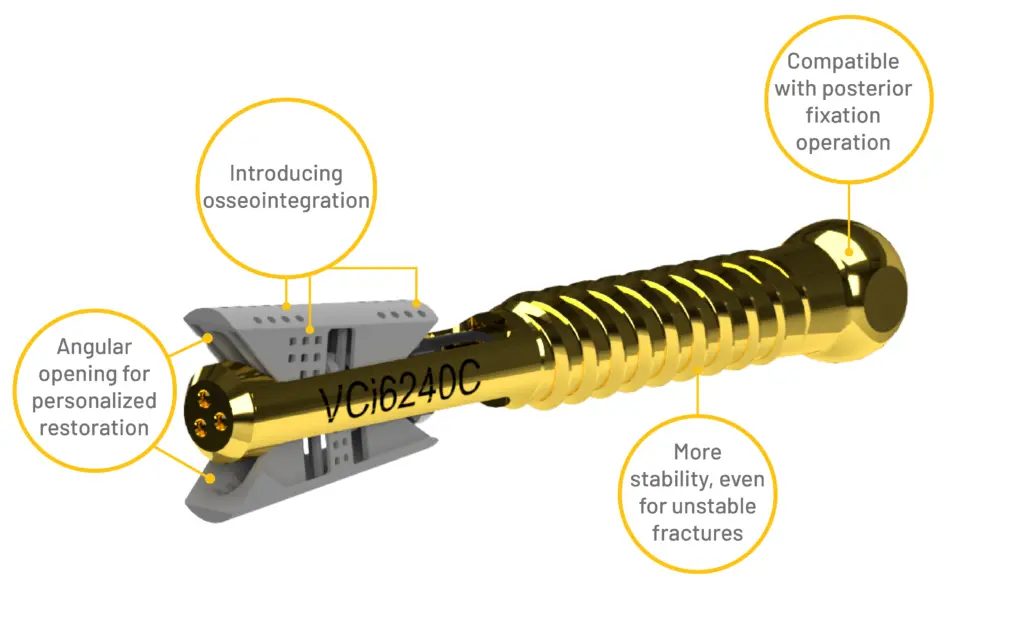
The VCFix® Spinal System is a revolutionary vertebral body augmentation system to improve pain control and stabilize the spine in patients suffering from Vertebral Compression Fractures.
The product information described on this page is specific to the United States of America. Switch to your country of preference to view the relevant information for that region.
VCFix® is a revolutionary implant for vertebral body augmentation developed by Amber Implants. We designed this product for the treatment of vertebral compression fractures. The product, consisting of a preparation, implantation, and an optional supplementary fixation kit, is intended to be used to treat and reduce compression fractures in vertebrae that have collapsed due to trauma, tumor, or degenerative diseases.
VCFix®: improving the treatment of spinal fractures
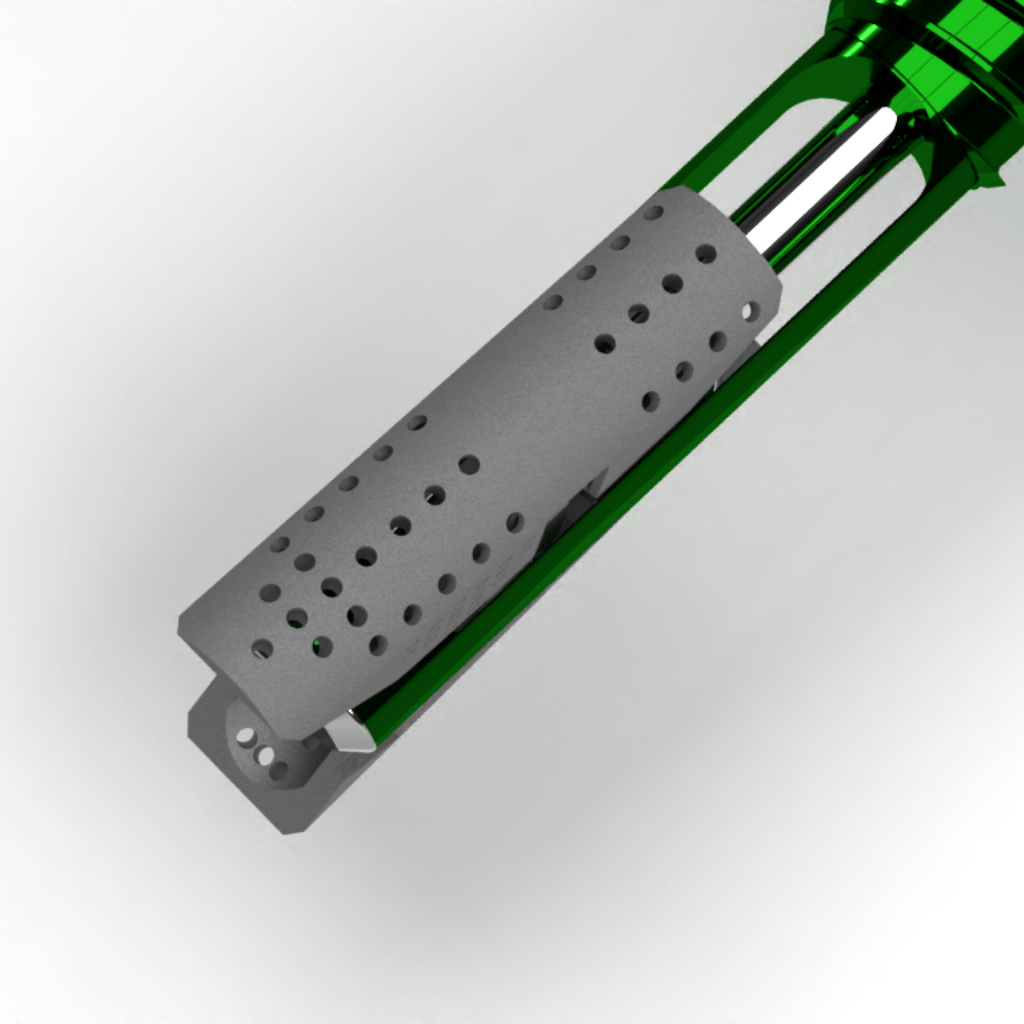
Inducing osseointegration
Due to its optimized perforated structure and appropriate mechanical properties, VCFix® offers an ideal bone-implant interface.
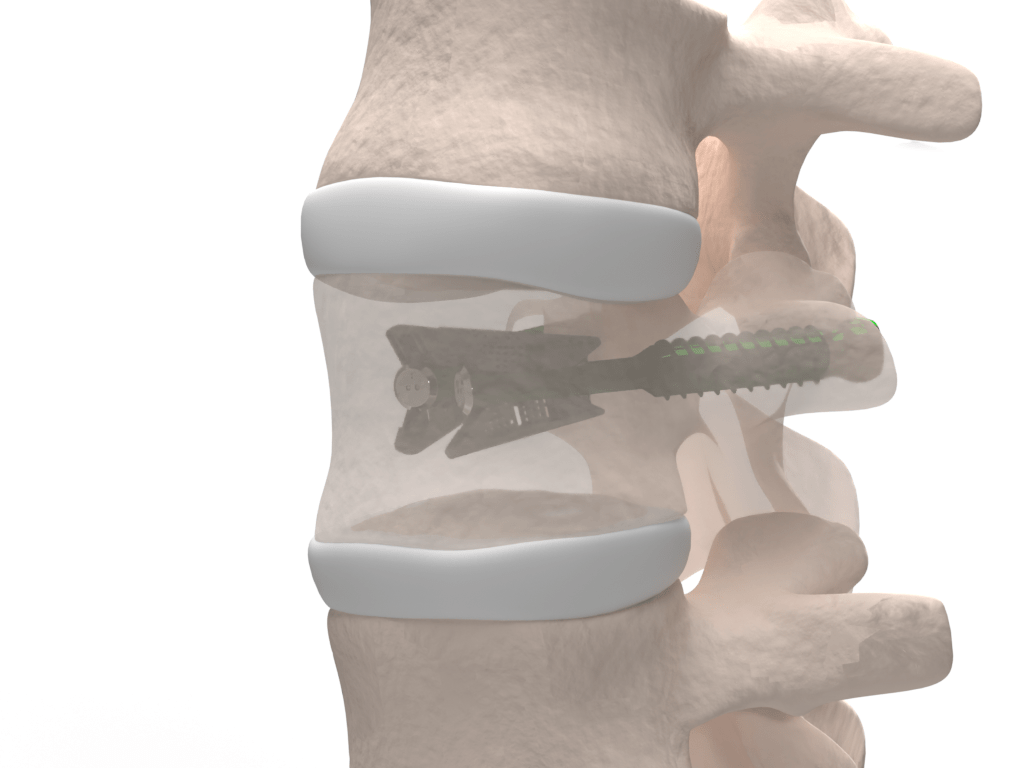
Stand-alone device
Thanks to its robustness, VCFix® allows for the treatment of vertebral compression fractures without the need for bone cement. However, for more severe and unstable fractures, the implant is compatible with cement injection.
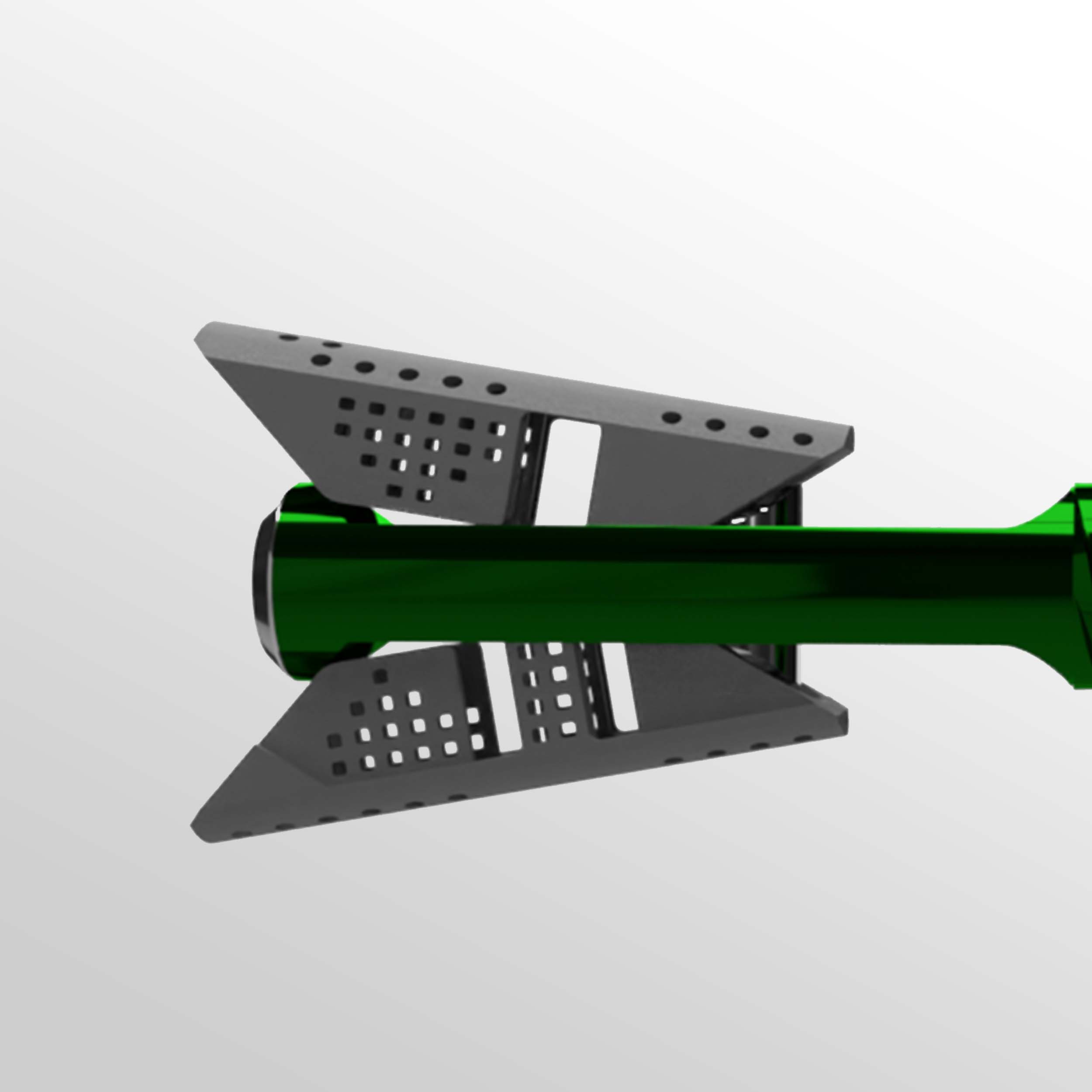
Controlled restoration
VCFix® implant comes in different sizes and has an adjustable angular expansion mechanism. This way, VCFix® provides an optimal fracture reduction and anatomical restoration for each patient.
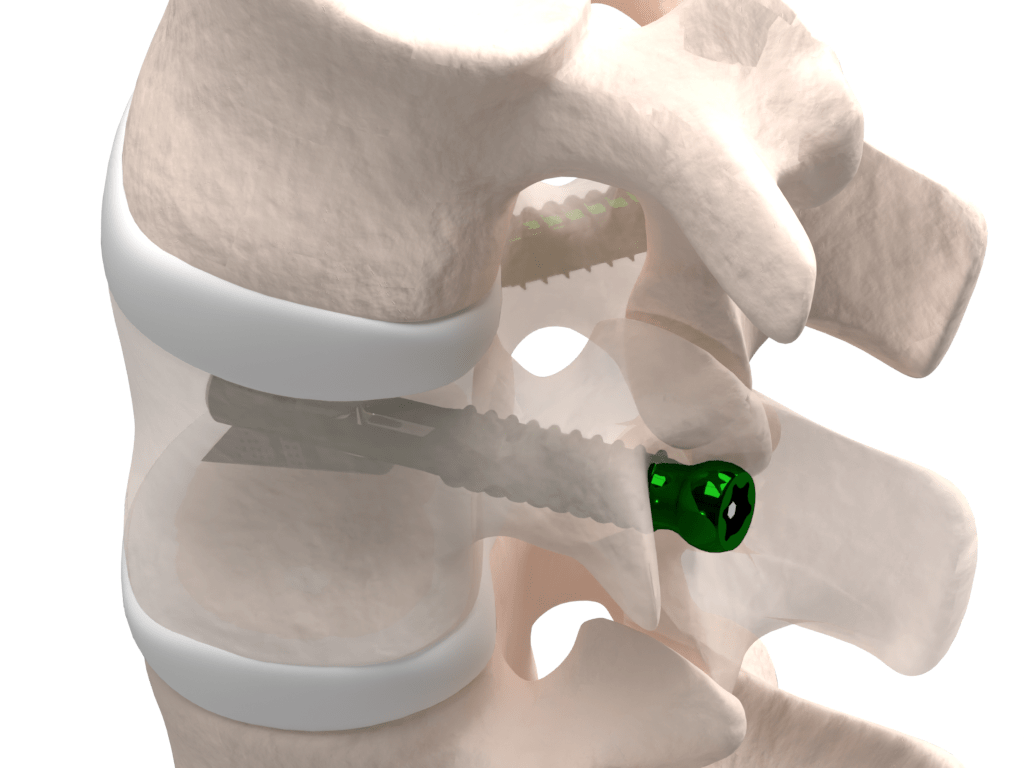
Better stabilization
VCFix® provides pedicle anchorage to better distribute the biomechanical load on the vertebral body and the spine. In the case of more complex fractures, posterior fixation is an optional feature.