The VCFix® Spinal System is a revolutionary vertebral body augmentation system to improve pain control and stabilize the spine in patients suffering from Vertebral Compression Fractures.
CAUTION Investigational device. Limited by Federal law to investigational use.
The product information described on this page is specific to the United States of America. Switch to your country of preference to view the relevant information for that region.
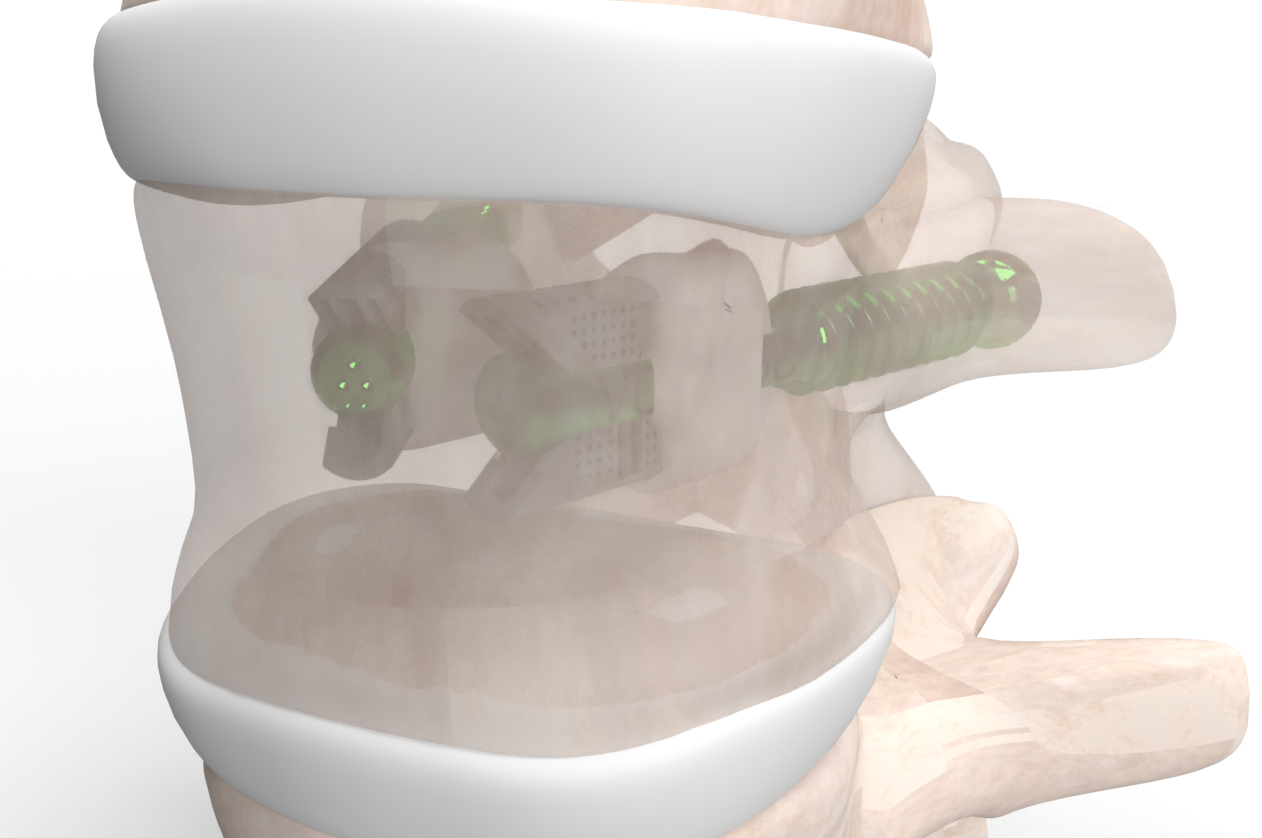
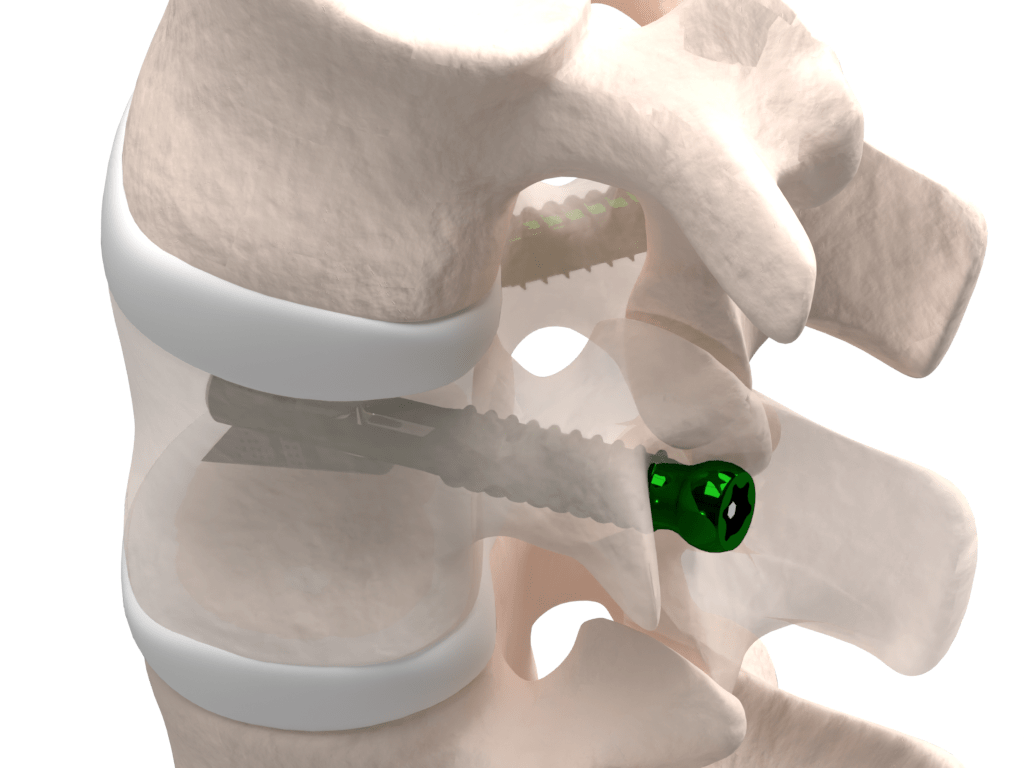
VCFix® is a revolutionary implant for vertebral body augmentation developed by Amber Implants. We designed this product for the treatment of vertebral compression fractures. The VCFix® Spinal System is intended to be used in combination with an approved bone cement, and to be placed, using a transpedicular approach, in a fractured vertebra with or without posterior fixation.
VCFix®: improving the treatment of spinal fractures
Optimum mechanical performance
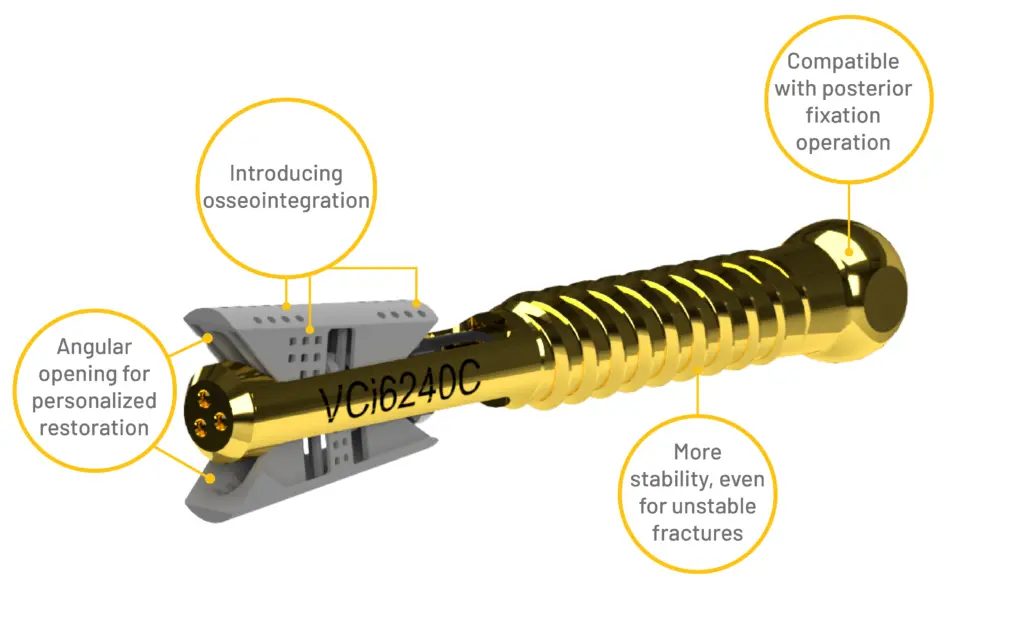
VCFix® expands in a craniocaudal direction, tackling the compression forces of the spine with up to 1200 newtons of expansion force. Furthermore, after the reduction of the fracture, they can carry more than 4000N without yielding. These results create confidence in the performance and safety of our device for treating a broad range of vertebral compression fractures
Controlled restoration
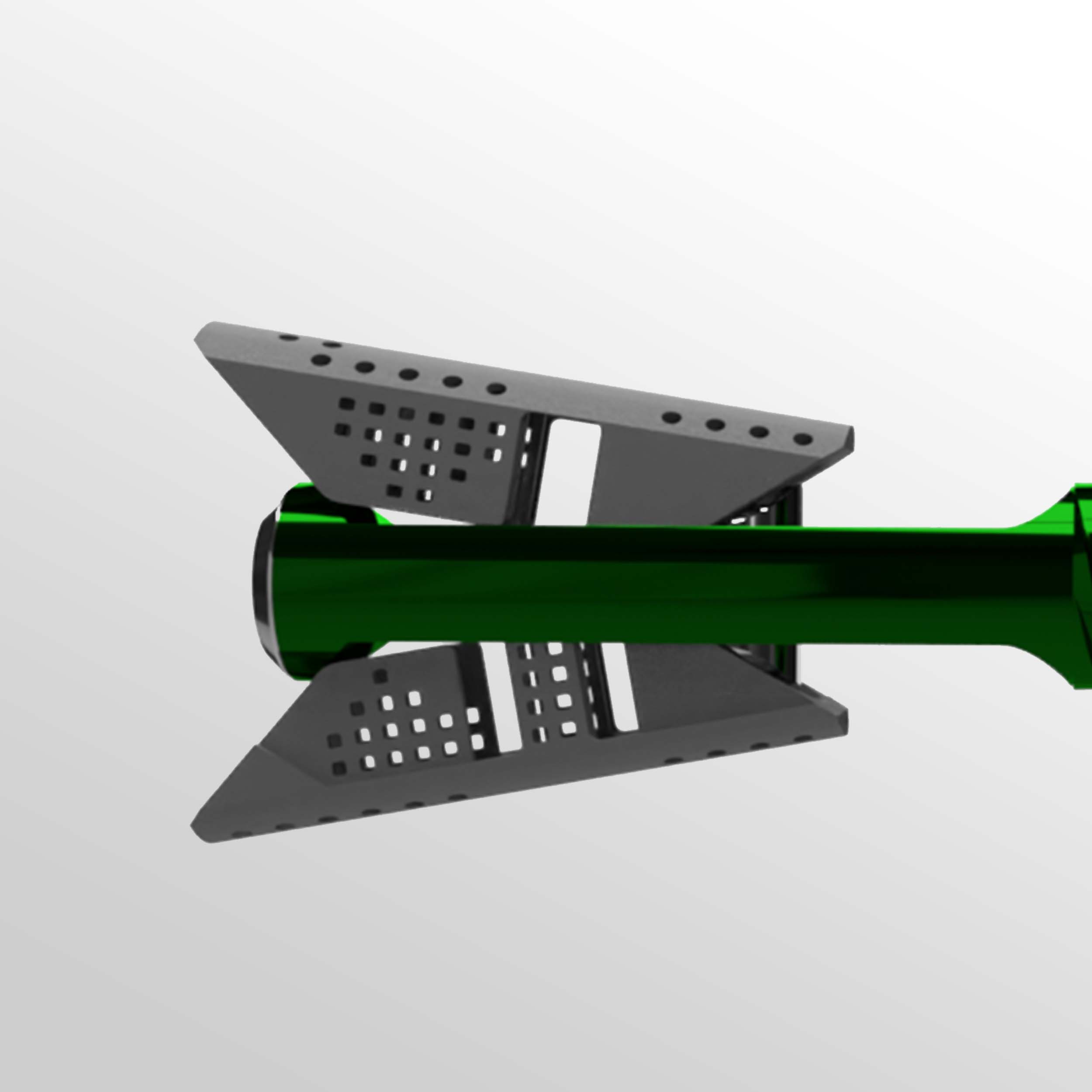
VCFix® implant comes in different sizes and has an adjustable angular expansion mechanism. This way, VCFix® provides an optimal fracture reduction and anatomical restoration for each patient.
Better stabilization
VCFix® provides pedicle anchorage to better distribute the biomechanical load on the vertebral body and the spine.
Future Developments
VCFix® Spinal System is still under development. We are continuously working on improvements to the systems to ensure the best possible treatment for spinal injuries. The validations of the developments we describe below are still ongoing.
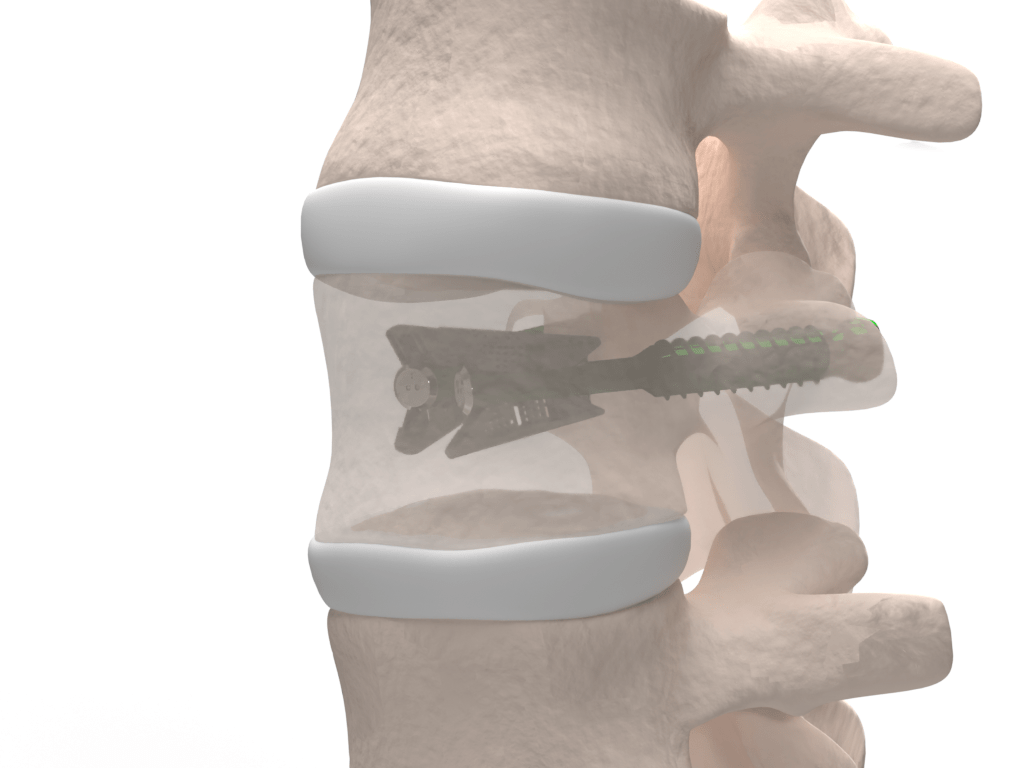
Minimize cement
We are optimizing VCFix® implant to treat vertebral fractures as a stand-alone device. This means that in the future it can be used with the minimum amount of cement possible and therefore avoid any risk related to cement leakages.
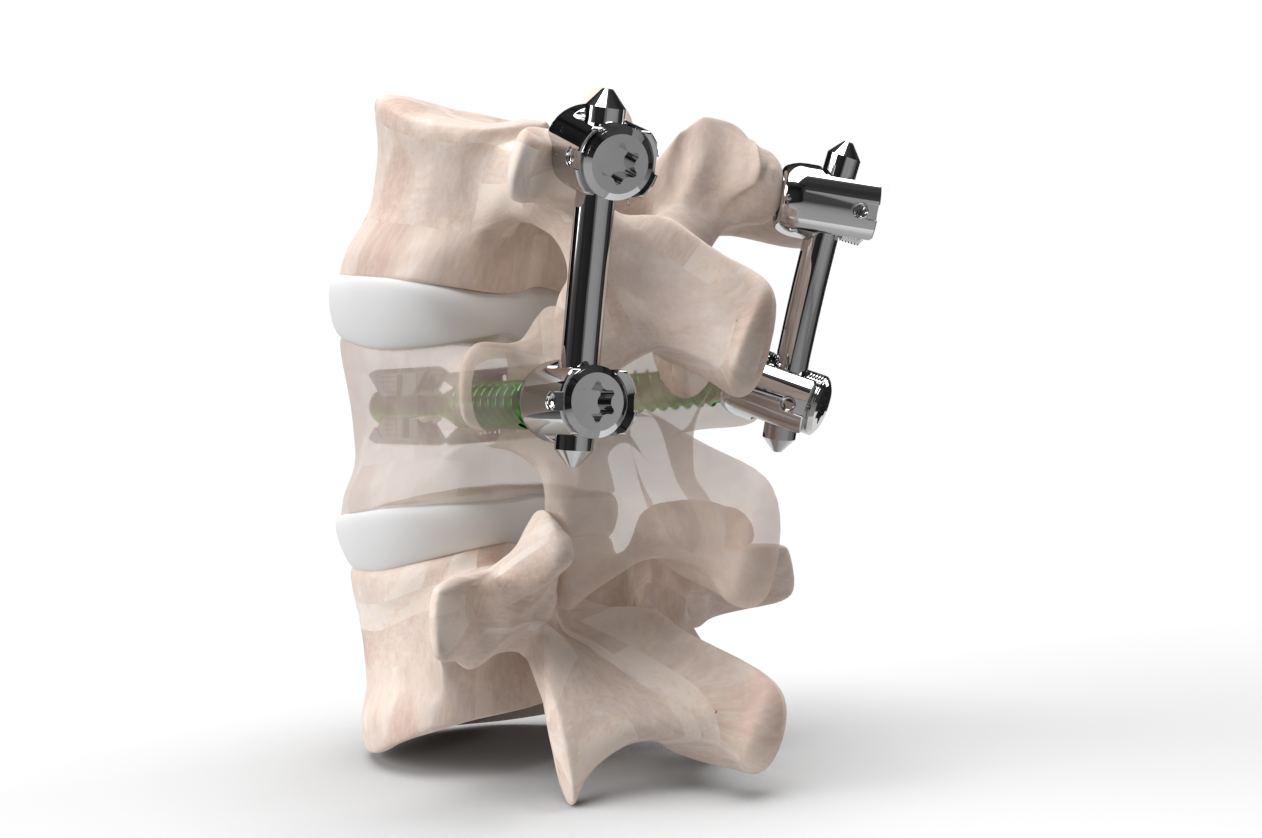
Compatibility with posterior fixation
VCFix® is compatible with both short and long-segment posterior fixation. This design feature enables the treatment of incomplete burst fractures through single-level fixation located cranial to the fracture, thereby preserving an intact mobile segment that would otherwise need immobilization.
VCFix® system timeline
Start of the clinical trials
Regulatory activities to prepare for product launch in the US.
Introduction of VCFix Spinal System with cement in the US market.