The VCFix® Spinal System is continuously evolving to meet more patient needs. We are currently developing new products and product features that allow us to expand the indications of our system. Amber Implants aims to provide advanced solutions for an ever-broader range of vertebral fractures.
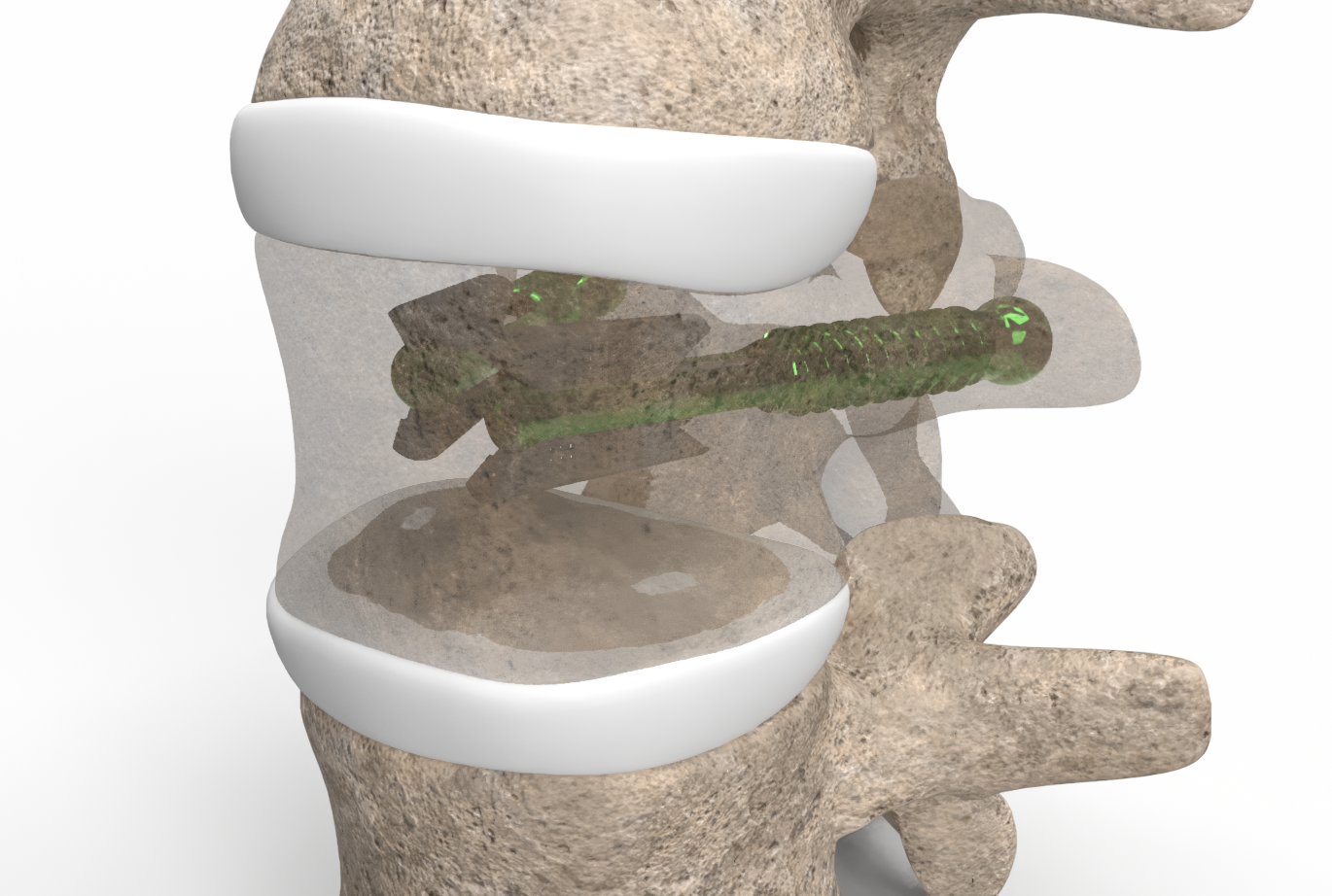
VCFix Standalone
We are currently clinically investigating the VCFix® implant as a stand-alone device for treating vertebral fractures VCFix® implant to treat vertebral fractures as a stand-alone device. This aim is to validate that the mechanical strength of the VCFix Implant is sufficient to be used without cement. This will reduce operating times even more and avoid any risk related to cement leakages.
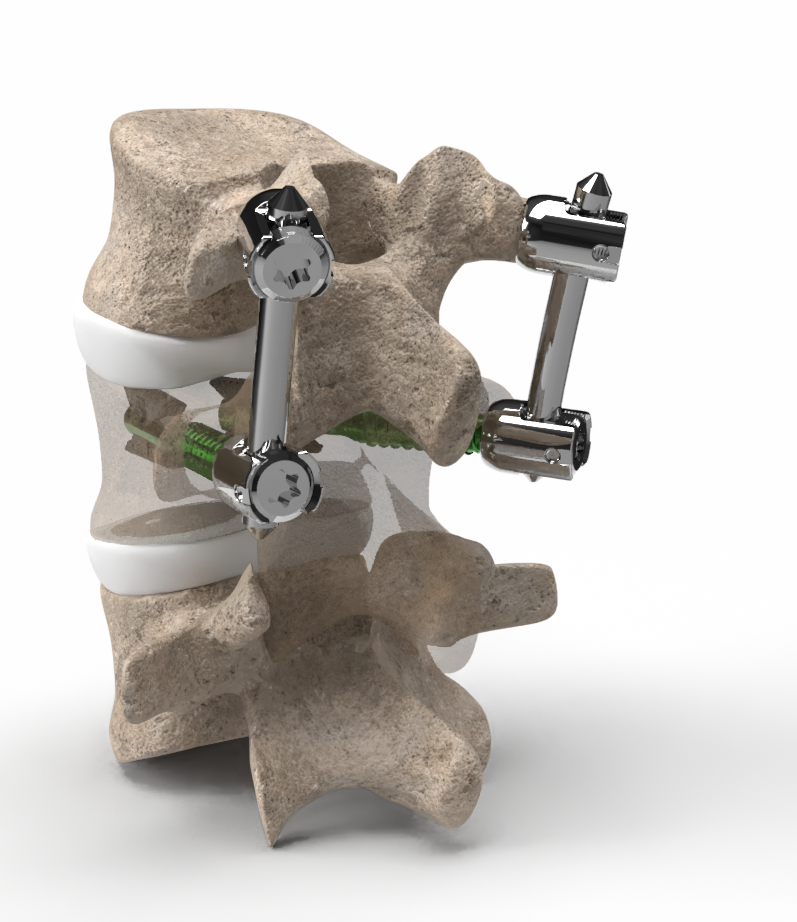
Compatibility with posterior fixation
We are currently developing products that make VCFix® compatible with both short and long-segment posterior fixation. This design feature enables the treatment of incomplete burst fractures through single-level fixation located cranial to the fracture, thereby preserving an intact mobile segment that would otherwise need immobilization.

Compatibility with posterior fixation
We are currently developing products that make VCFix® compatible with both short and long-segment posterior fixation. This design feature enables the treatment of incomplete burst fractures through single-level fixation located cranial to the fracture, thereby preserving an intact mobile segment that would otherwise need immobilization.
VCFix® System Pipeline
Planned submissions to obtain FDA and CE marking for the label expansion of VCFix stand-alone.
Planned submissions to obtain FDA and CE marking for the label expansion of VCFix Spinal System with posterior Fixation®
Validation for the compatibility of VCFix® Spinal System with posterior fixation and clinical trials (EXPAND study) with VCFix® Stand-alone
Validation for the compatibility of VCFix® Spinal System with posterior fixation and clinical trials (EXPAND study) with VCFix® Stand-alone
