We’re looking forward to attending the American Society of Spine Radiology (ASSR) 2026 Annual Meeting, February 26-28, in New Orleans, LA. As we expand into...
Read MoreRead all about the latest events and news items related to Amber Implants on this page. Also, you can find al our scientific publications and white papers at the bottom of the page for more in depth information on our activities. Follow us on LinkedIn for more frequent updates.
EVENT: Upcoming Friday Amber Implant will attend the German Spine Congress
Amber Implants will attend the DWG Annual Meeting in Wiesbaden! We’re looking forward to attending the 20th Annual Meeting of the Deutsche Wirbelsäulengesellschaft (DWG) on...
Read MoreEVENT: Amber Implants will attend the NASS Annual Meeting in Denver
Amber Implants is heading to the 40th North American Spine Society Annual Meeting, Nov 14–16 in Denver. Our CEO, Vincent Gardès, and CTO, Mo Ahmadi,...
Read MoreNEWS: Amber Implants Secures FDA 510(k) Clearance for VCFix® Spinal System: A Next-Generation Solution for Vertebral Compression Fractures
The Hague, The Netherlands, 17 September 2025 – Amber Implants, an innovative medical technology company developing next-generation implants for spinal injuries, today announced it has...
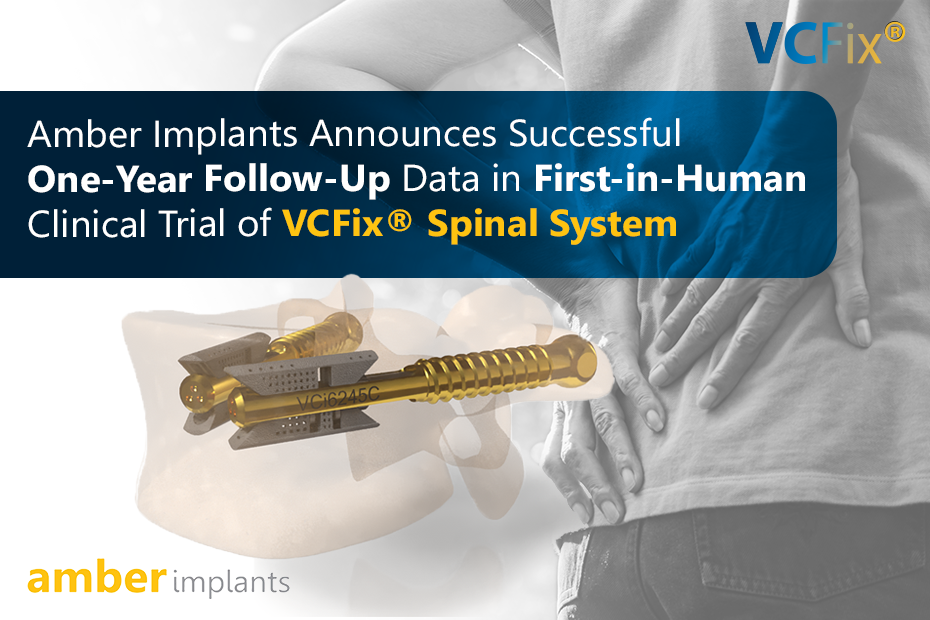
Read MoreNEWS: Amber Implants Announces Successful One-Year Follow-Up Data in First-in-Human Clinical of VCFix® Spinal System
The Hague, The Netherlands, 3 June 2025 – Amber Implants, an innovative medical technology company developing next-generation implants for spinal injuries, today announces the completion...
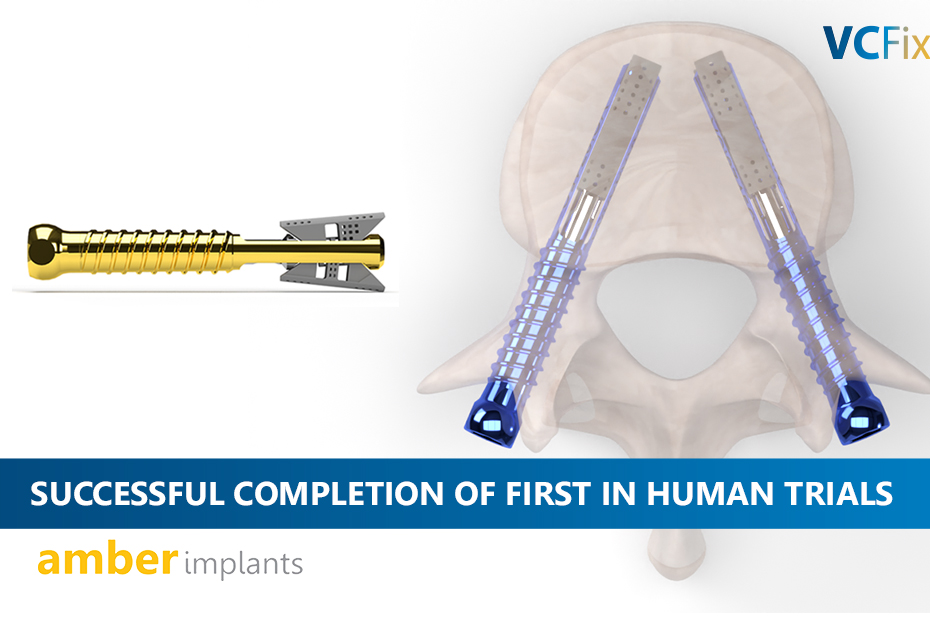
Read MoreNEWS: Amber Implants Completes Enrolment of First-In-Human Clinical Trial of its VCFix® Spinal System
The Hague, Netherlands, 11 June 2024: Amber Implants (‘Amber’ or ‘the Company’), an innovative medical technology company developing next-generation implants for spinal injuries, today announces...
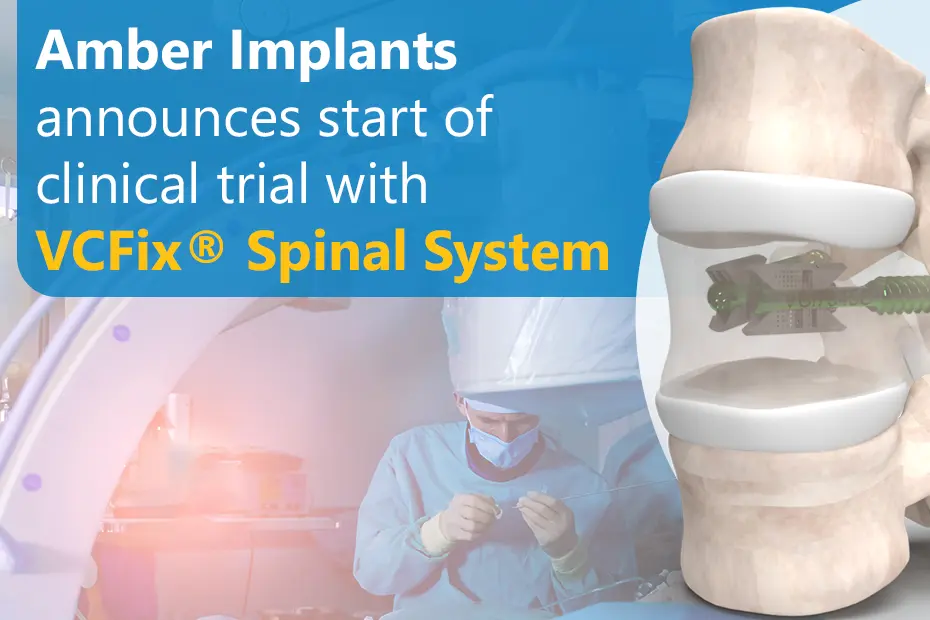
Read MoreNEWS: Amber Implants Announces Start of Clinical Trial with VCFix® Spinal System
Pioneering Safer and More Effective Solutions for Vertebral Fractures The Hague, Netherlands, 3 October 2023: Amber Implants, an innovative medical technology company developing next-generation implants...
Read MoreNews: Amber Implants receives ISO 13485:2016 certification.
The Hague, Netherlands, July 6, 2022: Amber Implants, an innovative medical technology company developing next generation spinal implants for spinal injuries, today announces that it has...
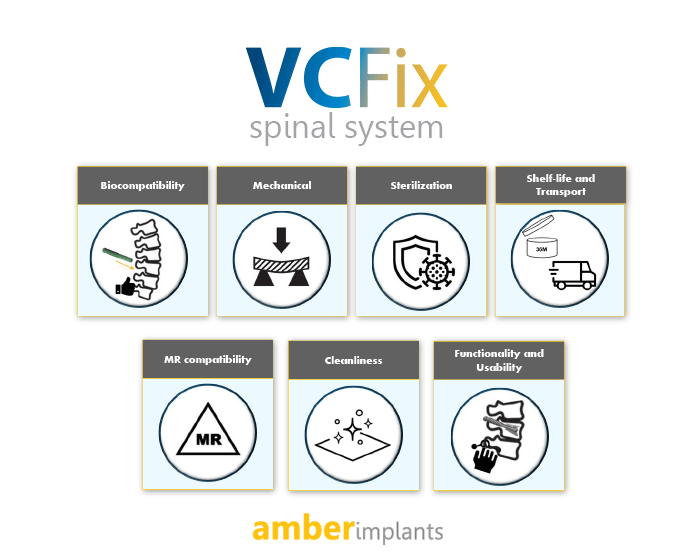
Read MoreNews: VCFix® Spinal system has passed all the preclinical tests required for clinical use of the device
Today is an exciting day for Amber Implants! We are proud to announce that our product, the VCFix® Spinal System, has successfully passed all preclinical...
Read MorePUBLICATION | Evaluation of biocompatibility and osseointegration of multi-component TiAl6V4 titanium alloy implants
Orthopaedic implants are crucial for restoring the function, stability, and mobility of bones, and they can be made from materials such as metal, ceramic, polymer,...
Read MoreWhitePaper: Bioplastics for 3D Printed Medical Implants
Polymers are particularly attractive in the medical device field due to their biocompatibility, mechanical properties comparable to those of the host tissues, and customizable manufacturing...
Read MoreWhitePaper: Metallic Bio-Materials that can be used for 3D printed Medical Implants
Currently more than 70% of clinically used implants are made from metallic materials, such as stainless steel, cobalt chromium alloys, titanium alloys and others. However,...
Read MoreWhitePaper: 5 Steps to set-up a solid quality management system for 3D printed implantable devices
Since medical devices directly affect the human body, safety is more important than non-medical industrial products. Each country has established standards and regulations to ensure...
Read More